Pan Chaoyun's research group of our hospital, in conjunction with Liu Junxiu and Li Jie's research group of gynecology department of Zhongshan First Hospital, reveals the metabolic regulation mechanism of ovarian cancer pelvic and abdominal metastasis
The research team led by Professor Pan Chaoyun from our hospital, in collaboration with the research teams led by Liu Junxiu and Li Jie from the Department of Gynecology, The First Affiliated Hospital of Sun Yat-sen University, has revealed the metabolic regulatory mechanism underlying the peritoneal metastasis of ovarian cancer. Peritoneal metastasis is a common metastatic pathway of ovarian tumors and is closely associated with poor prognosis in patients. Tumor cells often exhibit specific metabolic reprogramming during the metastatic process, which is expected to provide important targets for tumor therapy. On April 3, 2025, the team led by Pan Chaoyun from the Department of Biochemistry and Molecular Biology of our hospital, together with the teams led by Liu Junxiu and Li Jie from the Department of Gynecology, The First Affiliated Hospital, jointly published a paper entitled A noncanonical role of SAT1 enables anchorage independence and peritoneal metastasis in ovarian cancer in the journal Nature Communications. Based on experiments such as systematic screening of metabolic rate-limiting enzymes, epigenetic analysis, metabolomics, and biochemical analysis, the paper found that the polyamine metabolizing enzyme SAT1 (spermine/spermidine N1-acetyltransferase 1) is a key factor driving peritoneal metastasis of ovarian tumor cells, and targeting SAT1 is a potential therapeutic strategy for metastatic ovarian cancer.
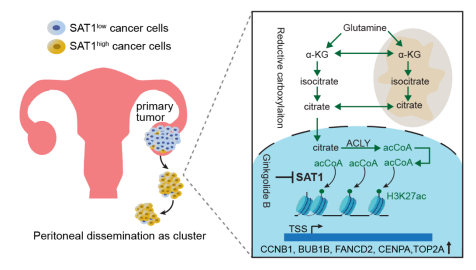
Original article link: https://www.nature.com/articles/s41467-025-58525-8
The study found that hypoxia induces high expression of SAT1, and SAT1 enhances peritoneal metastasis by promoting anchorage-independent survival of tumor cells. Cancer cells with high SAT1 expression are commonly present in malignant ascites. SAT1 directly acetylates the H3K27 domain of key mitotic regulatory genes, increases their transcription levels, and thereby protects anchorage-independent metastatic cells from mitotic catastrophe and cell death. The acetylation of H3K27 by SAT1 depends on acetyl-CoA provided by glutamine through the reductive carboxylation pathway. The study also found that ginkgolide B, a natural product with both medicinal and edible values, can effectively inhibit SAT1 activity and significantly reduce metastatic tumor burden in mouse models.
On February 14, 2025, Professor Pan Chaoyun's team, in collaboration with Liu Junxiu's and Li Jie's teams, also published a paper entitled ACAT1-Mediated ME2 Acetylation Drives Chemoresistance in Ovarian Cancer by Linking Glutaminolysis to Lactate Production in Advanced Science. This paper reveals that under chemotherapy pressure, ovarian tumor cells produce lactate through glutamine metabolism, thereby promoting DNA damage repair and chemoresistance, providing a new perspective on the metabolic regulatory mechanism of chemoresistance.
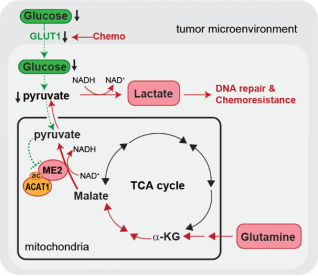
Original article link: https://advanced.onlinelibrary.wiley.com/doi/full/10.1002/advs.202416467
Ph.D. students Zheng Cuimiao, Lu Jingyi, and master student Huang Qian from Professor Pan Chaoyun's team are the co-first authors of the two research papers. Professor Pan Chaoyun, Professor Liu Junxiu, and Associate Researcher Li Jie are the corresponding authors. The research was supported by the Excellent Young Scientists Fund, General Program of the National Natural Science Foundation of China, and the Key Research and Development Program of the Ministry of Science and Technology. Animal experiments were carried out at the Laboratory Animal Center of Sun Yat-sen University. Sun Yat-sen University is the first affiliated unit of the papers.


