Circulation Research | Cai Weibin’s team reveals a new mechanism of metabolic-structure pathological connection in diabetic myocardial injury
The number of people with diabetes in China has reached 148 million, which has become a major public health issue seriously threatening national health and poses severe challenges to the "Healthy China 2030" strategy. The clinical application of insulin and the emergence of new treatment methods have prolonged the lifespan of diabetic patients, making the originally hidden or late-onset diabetic myocardial injury "move from behind the scenes to the front stage", which has become an important risk factor for the death of diabetic patients. Lipid metabolism disorder and abnormal myocardial structure are two typical pathological features of diabetic myocardial injury, but the mechanism by which chemical metabolism disorder leads to physical structural abnormalities, and how metabolism and structure are coupled to synergistically promote diabetic cardiac dysfunction has not been clarified. Systematically analyzing the action mode and molecular mechanism of metabolic-structural pathological connections is expected to provide new strategies and intervention targets for the precise treatment of diabetic cardiomyopathy, and offer Chinese wisdom for cardiovascular protection in diabetic patients worldwide.
On April 7, 2025, a collaborative team led by Professor Cai Weibin from the Zhongshan School of Medicine and the Laboratory Animal Center of Sun Yat-sen University, together with Professor Chen Hui's team from the Reproductive Medicine Center of the Sun Yat-sen Memorial Hospital of Sun Yat-sen University, published a research paper entitled Metabolic Coordination Structures Contribute to Diabetic Myocardial Dysfunction in the renowned journal Circulation Research. The study utilized techniques such as metabolic flux and omics analysis, and three-dimensional transmission electron microscopy to observe that ACBP inhibits the myocardial contractile apparatus by binding to the key sarcomere structural protein MyBPC3, explaining a new mechanism of diabetic myocardial injury from the perspective of metabolic-structural pathological connections.
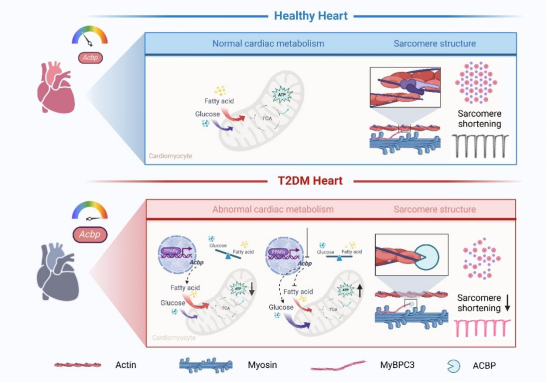
Through a multi-dimensional research system, the team found that the hearts of diabetic mice with systolic and diastolic dysfunction exhibit enhanced lipid metabolism and impaired ultrastructure. By analyzing 4 public transcriptome datasets of type 2 diabetes mellitus (T2DM) myocardium, the authors found that acyl-CoA binding protein (ACBP) is an important lipid metabolism gene that is upregulated in diabetic myocardium. The team constructed cardiomyocyte-specific ACBP knockout mice (α-MyHC-Cre:Acbpflox/flox mice), which showed alleviated T2DM-induced cardiac remodeling and cardiac dysfunction. Further using 13C-labeled glucose and palmitic acid for isotope-labeled metabolic flux tracing analysis, it was found that glucose oxidation was enhanced and fatty acid utilization was reduced in the hearts of α-MyHC-Cre:Acbpflox/flox mice. ACBP deficiency improved myocardial energy supply through metabolic reprogramming and metabolic compensation in diabetic myocardium.
To further explore the molecular basis of metabolism and structure coupling, the team used Co-IP combined with mass spectrometry and found that ACBP directly binds to the myocardial cytoskeletal structural protein MyBPC3. Three-dimensional reconstruction of high-resolution transmission electron microscopy showed that the ACBP-MyBPC3 interaction hindered the formation of cross-bridge structures, leading to a reduction in thick/thin filament cross-linking structures and inhibiting myocardial contraction. Meanwhile, ChIP-seq and Luciferase Assay confirmed that the PPARγ-ACBP axis is a key regulatory pathway for metabolic-structural coupling in diabetic myocardium.
This study is the first to propose that "metabolic-structural coupling" is an important pathogenesis of diabetic cardiomyopathy. Under diabetic conditions, ACBP can serve as a dual-effect target to simultaneously improve myocardial metabolic and structural abnormalities. Targeted intervention of ACBP can simultaneously achieve myocardial metabolic remodeling (restoring substrate metabolic flexibility) and structural repair (relieving ACBP-MyBPC3 pathological interaction), which is expected to become a potential therapeutic target for improving DCM.
Professor Cai Weibin and Associate Researcher Tan Jing from the Zhongshan School of Medicine of Sun Yat-sen University, and Professor Chen Hui from the Reproductive Medicine Center of the Sun Yat-sen Memorial Hospital of Sun Yat-sen University are the co-corresponding authors of the paper. Wu Teng, a 2020-level doctoral student from the Zhongshan School of Medicine (now a postdoctoral fellow at the Sun Yat-sen Memorial Hospital), and Huang Tongsheng, a 2019-level doctoral student, are the co-first authors. The Laboratory Animal Center of Sun Yat-sen University/Guangdong Provincial Engineering Technology Research Center for Disease Model Animals, Professor Huang Peng's team from the Metabolomics Platform of the Zhongshan School of Medicine, Associate Professor Zhang Qinfen's team from the Electron Microscopy Platform of the School of Life Sciences, and the Scientific Instrument Platform of the Zhongshan School of Medicine provided important technical support for this research.
Original article link: https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.124.326044


