Activities
A newly-published review in Cell Regeneration 在Cell Regeneration新发表综述1篇
Link to the review:
https://cellregeneration.springeropen.com/articles/10.1186/s13619-022-00145-4
https://pubmed.ncbi.nlm.nih.gov/36539553
Cell fate transitions are accompanied by dynamics of 3D chromatin organization and phase separation. In recent years, the development of technologies detecting chromatin interactions has facilitated the study of 3D chromatin structure. In the crowded nuclear space, chromatin folds to form specific hierarchical structures: A/B compartment, topologically associating domain and chromatin loop. Dramatic changes in chromatin folding state during cell fate transition are closely related to gene expression differences. The formation mechanism and dynamic pattern of 3D chromatin structure are important for us to better understand how gene expression and cell fate transition are regulated.
Phase separation is the main mechanism for the formation of intracellular biomolecular condensates, i.e., biomolecules such as proteins and nucleic acids form aggregates that are not dependent on cell membrane through weak multivalent interactions. Classical phase separation include membraneless organelles, such as germ granules, Cajal bodies, and condensates formed by transcription-associated factors bound to DNA, etc., which play important biological functions. Phase-separated biomolecular condensates undergo fusion, separation, and changes in size, localization and composition during cell fate transitions. These interesting changes conceal unknown mechanisms of cell fate regulation.
Several studies have shown that phase separation is involved in the regulation of 3D chromatin organization. Transcription factors can facilitate the reconstruction of 3D chromatin structure via phase separation, and thus regulate cell fate transition. Abnormal phase separation in the nucleus lead to chromatin misfolding and promote the development of diseases. Therefore, an in-depth study of the relationship between 3D chromatin organization and phase separation can help reveal the mechanisms of cell fate regulation in both physiological and pathological states.
On 21st December 2022, the review of Professor Junjun Ding's team entitled "The dynamics of three-dimensional chromatin organization and phase separation in cell fate transitions and diseases" is published in Cell Regeneration, which systematically reviews the dynamics of 3D chromatin organization and phase separation in various cell fate transitions and diseases, and highlights direct and indirect evidence for the involvement of phase separation in regulating chromatin folding. Based on these, strategies to study the relationship between 3D chromatin organization and phase separation are summarized. Besides, a model of how 3D chromatin organization and phase separation jointly or separately regulate gene expression and further affect cell fate transitions is proposed.
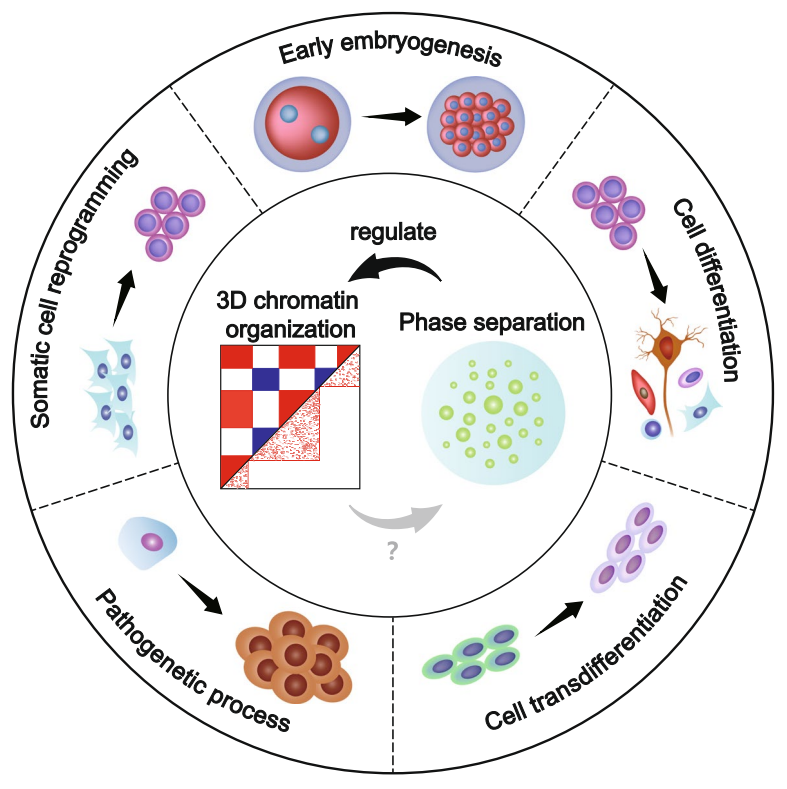
By reviewing recent studies, this work summarizes that 3D chromatin organization may undergo dynamics of certain patterns during specific cell fate transition. For example, the proportion and internal interactions of B compartment increase, the number of TADs decreases, and cell type-specific enhancer-promoter interactions are established during mammalian stem cell differentiation.
Studies focusing on the relationship between phase separation and 3D chromatin organization are divided into direct and indirect evidence. Direct evidence refers to studies that can strongly support the regulatory relationship, mainly using the following three research strategies: (1) artificially induce phase separation at specific genomic loci; (2) disrupt all phase separation in the nucleus by using 1,6-hexanediol (1.5%, 2 min); (3) disrupt the phase-separating property of target proteins by mutation and restore their phase separation by fusing intrinsic disordered regions, then observe the changes of 3D chromatin organization. Indirect evidence refers to studies suggesting a correlation between the two and can be divided into (1) known nuclear phase separation are involved in regulating 3D chromatin organization, (2) chromatin structural factors undergo phase separation, (3) heterochromatin-associated proteins or non-coding RNAs promote chromatin condensation in the form of phase separation, and (4) computational method reveals that phase separation can drive chromatin folding.
In conclusion, phase separation is one of the most important mechanisms regulating the 3D chromatin organization. Studying the relationship between 3D chromatin organization and phase separation can help us further understand how cell fate is regulated. How they are involved in cell fate regulation can be briefly summarized in the following three models: (1) phase separation affects cell type-specific gene expression to regulate cell fate by regulating 3D chromatin structure at different hierarchies, (2) specific chromatin structure recruits different phase separations, affects cell type-specific gene expression to regulate cell fate, (3) phase separation and 3D chromatin structure affect cell type-specific gene expression to regulate cell fate without interfering with each other. There are still many questions about 3D chromatin structure and phase separation remaining to be solved. It is believed that a more comprehensive and clear mechanism will be demonstrated in the near future.
The review was co-authored by Xiaoru Ling, undergraduate, and Xinyi Liu, Ph.D Candidate, Zhongshan School of Medicine. Junjun Ding, Professor of Zhongshan School of Medicine at Sun Yat-Sen University, is the sole corresponding author.
细胞命运转变的过程中伴随着染色质三维结构3D chromatin organization与相分离phase separation的动态变化。近年来,染色质互作检测技术的发展促进了染色质三维结构的研究。在拥挤的细胞核空间中,染色质会折叠形成特定的层级结构:A/B compartment, topologically associating domain以及chromatin loop。在细胞命运转变过程中,染色质折叠状态的剧烈改变与基因表达的变化密切相关。确定染色质三维结构的形成机制与变化规律对于理解基因表达调控、细胞命运转变具有重要意义。
相分离是细胞内生物分子聚集物形成的主要机制,即蛋白质、核酸等生物大分子通过弱的多价相互作用形成不依赖于生物膜的聚集体。经典的相分离现象包括germ granules、Cajal bodies等无膜细胞器,以及转录调控相关因子与DNA结合形成的复合物等等,它们发挥着非常重要的生物学功能。在细胞命运转变过程中,相分离的生物分子聚集物会经历融合,分离,以及大小、空间定位、组分等改变,这些有趣的变化中则隐藏着细胞命运调控的未知机制。
多项研究表明相分离是染色质三维结构的重要调控因素之一。转录因子可以通过相分离来促进染色质三维结构的重建,进而控制细胞命运转变。而细胞核内异常蛋白的相分离则会导致染色质的错误折叠,并促进疾病的发生发展。因此,深入研究染色质三维结构与相分离的相互调控关系有助于揭示生理和疾病状态下的细胞命运调控机理。
2022年12月21号,中山大学丁俊军课题组在Cell Regeneration发表了题为“The dynamics of three-dimensional chromatin organization and phase separation in cell fate transitions and diseases”的综述,系统回顾了染色质三维结构以及相分离在多种细胞命运转变与疾病过程中的动态变化,着重阐述了相分离参与调控染色质三维结构形成的直接与间接证据,并在此基础上总结了目前研究二者关系的策略, 提出染色质三维结构与相分离是如何共同/分别调控基因表达并影响细胞命运转变的模型。
本综述通过整理和比较近年来的多项研究,发现在特定的细胞命运转变过程中染色质三维各层级结构的改变具有一定的规律性。以哺乳动物干细胞分化为例,总体来说,B compartment的比例在细胞分化后增多且内部互作增强,TAD的数目减少,细胞类型特异性的增强子-启动子互作建立。
围绕相分离参与调控染色质三维结构,将已有的相关研究分为直接证据和间接证据。直接证据是指能够充分证明调控关系的研究,使用的研究策略主要有以下3种:(1)在特异的基因组位点上人为诱导相分离;(2)利用1,6-己二醇(1.5%,2 min)破坏细胞核内所有的相分离;(3)通过突变破坏目标蛋白的相分离能力,并通过融合内在无序区重新恢复其相分离能力,观察相分离能力的有无对染色质三维结构的影响。间接证据是指仅能证明两者相关联的研究,可归类为(1)已知的核内相分离结构参与调控染色质三维结构;(2)染色质结构因子具有相分离能力;(3)异染色质相关蛋白或非编码RNA以相分离的形式促进染色质凝集;(4)计算机建模发现相分离可以驱动染色质三维结构的重建。
总结来说,相分离是调控染色质三维结构形成的重要机制之一。通过研究染色质三维结构与相分离的相互关系可以帮助我们更好地理解细胞命运是如何被调控的。二者如何参与细胞命运调控可简单概括为以下3种模型:(1)相分离通过调节染色质各层级结构的形成调控细胞类型特异性基因的表达,从而促进细胞命运转变;(2)特定的染色质三维结构通过促进相分离的形成调控细胞类型特异性基因的表达;(3)染色质三维结构与相分离各自调控细胞类型特异性基因的表达。当然,关于染色质三维结构与相分离,仍然有许多问题需要深挖并得到解决。相信在不久的将来,会有更加全面、明确的机制解析。
丁俊军课题组的本科生凌晓茹和博士研究生刘心仪为本文共同第一作者。丁俊军教授是本文的唯一通讯作者。
